Animal Fats And Plant Oils Differ Primarily In What Way?
nine.one Terminology for Vegetable Oils and Fauna Fats
Fat is a generic term for lipids, a grade of compounds in biochemistry. You would know them as greasy, solid materials plant in animal tissues and in some plants – oils that are solids at room temperature.
Vegetable oil is the fat extracted from plant sources. Nosotros may be able to excerpt oil from other parts of a establish, but seeds are the chief source of vegetable oil. Typically, vegetable oils are used in cooking and for industrial uses. Compared to water, oils and fats have a much higher boiling point. However, there are some plant oils that are non good for human consumption, as the oils from these types of seeds would require additional processing to remove unpleasant flavors or even toxic chemicals. These include rapeseed and cottonseed oil.
Animal fats come from different animals. Tallow is beefiness fat and lard is pork fatty. In that location is also chicken fatty, blubber (from whales), cod liver oil, and ghee (which is a butterfat). Animal fats tend to take more free fatty acids than vegetable oils do.
Chemically, fats and oils are too called "triglycerides." They are esters of glycerol, with a varying blend of fatty acids. Figure 9.1 shows a generic diagram of the structure without using chemical formulas.
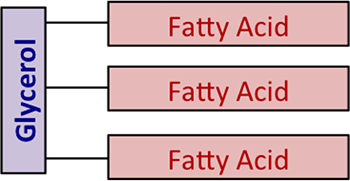
Figure nine.1: A generic diagram of oils and fats; a free fatty acid is when the fatty acid separates from the glycerol.
Credit: BEEMS Module B4
Then what is glycerol? Information technology is also known as glycerin/glycerine. Other names for glycerol include: one,2,3-propane-triol, 1,two,iii-tri-hydroxy-propane, glyceritol, and glycyl alcohol. It is a colorless, odorless, hygroscopic (i.e., will attract h2o), and sugariness-tasting pasty liquid. Effigy 9.2 shows the chemical construction in two different forms.
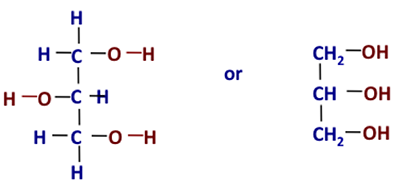
Figure 9.2: Chemic structure of glycerol.
Credit: BEEMS Module B4
And so now we demand to define what the fatty acids are. Essentially, fatty acids are long-concatenation hydrocarbons with a carboxylic acid. Effigy 9.3a shows the generic chemic structure of a fat acid with the carboxylic acid on it.

Effigy 9.3a: Generic carboxylic acid chemical structure.
Credit: BEEMS Module B4
Figure 9.3b shows different fat acid chemical structures. The chemical structures are shown every bit line chemical structures, where each point on the links is a carbon atom and the right number of hydrogen atoms is dependent on whether at that place is a single or double bail. Fatty acids can be saturated (with hydrogen bonds) or unsaturated (with some double bonds betwixt carbon atoms). Because of the metabolism of oilseed crops, naturally formed fatty acids contain fifty-fifty numbers of carbon atoms. In organic chemistry, carbon atoms accept four pairs of electrons bachelor to share with another carbon, hydrogen, or oxygen atom. Free fatty acids are not bound to glycerol or other molecules. They tin exist formed from the breakdown or hydrolysis of a triglyceride.
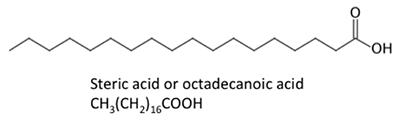
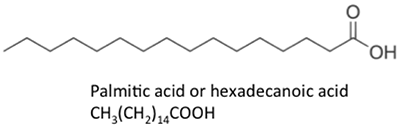
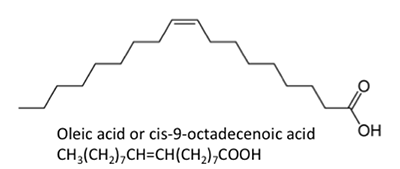
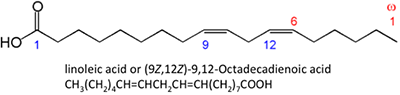
Figure 9.3b: Other long-chain acids, such as steric, palmitic, oleic, and linoleic acids.
Credit: BEEMS Module B4
The fatty acids shown have slightly unlike properties. Palmitic acid is found in palm oil. Figure 9.iv shows the relationship of each fat acrid to its size and saturation. Palmitic and steric acids are saturated fatty acids, while oleic and linoleic acids are unsaturated with different amounts of double bonds. Figure 9.4 shows differing amounts of carbon atoms compared to the number of double bonds in the compound.
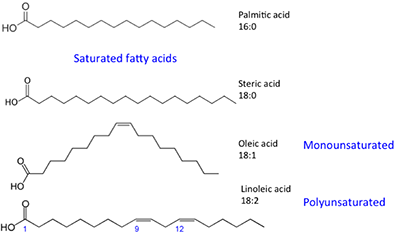
Effigy ix.4: Series of fat acids. The ratio represents the carbon atoms: double bonds in compound.
Credit: BEEMS Module B4
Figure 9.5a shows the part of the triglyceride that is a fatty acid and the part that is glycerol, including chemical structures this fourth dimension. The chemical structure shown here is a saturated triglyceride.
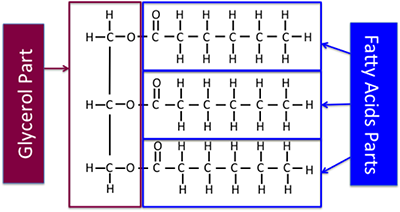
Figure 9.5a: Chemical construction of triglyceride, pointing out fat acid parts and glycerol part.
Credit: BEEMS Module B4
Then, we've discussed what fats and oils are. At present, what is biodiesel? What is at least one definition? It is a diesel fuel that was generated from biomass. However, there are different types of biodiesel. The most commonly known type of biodiesel is a fuel comprised of mono-alkyl esters (typically methyl or ethyl esters) of long-chain fat acids derived from vegetable oils or animal fats – this is according to ASTM D6551. An ASTM is a document that contains the standards for detail types of chemicals, peculiarly industrial materials. This is a wordy definition that doesn't really bear witness u.s.a. what it is chemically.
Then when we talk about an alkyl group, information technology is a univalent radical containing only carbon and hydrogen atoms in a hydrocarbon concatenation, with a general atomic formula of CnH2n+1. Examples include:
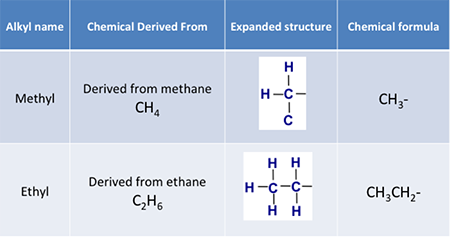
Effigy 9.5b: Alkyl groups divers for methyl and ethyl groups.
Credit: BEEMS Module B4
Another term nosotros demand to know nearly is anester. Esters are organic compounds where an alkyl group replaces a hydrogen atom in a carboxylic acid. For example, if the acid is acetic acrid and the alkyl grouping is the methyl grouping, the resulting ester is called methyl acetate. The reaction of acetic acid with methanol will form methyl acetate and water; the reaction is shown below in Figure 9.6. An ester formed in this method is a condensation reaction; it is also known as esterification. These esters are likewise called carboxylate esters.
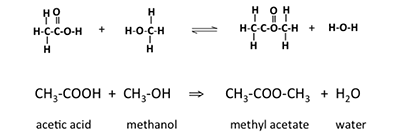
Effigy ix.6: Reaction of acetic acid with methanol to form methyl acetate and water.
Credit: BEEMS Module B4
This is the basic reaction that helps to form biodiesel. Figure 9.7 shows the different parts of the chemical construction of the biodiesel, the methyl ester fatty acid, or fat acid methyl ester (FAME).
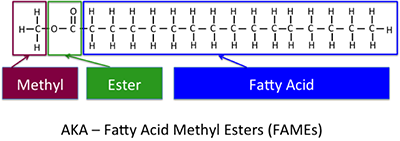
Figure 9.7: The chemical structure of the typical biodiesel, methyl ester fatty acid, or FAME.
Credit: BEEMS Module B4
Then, at this point, let'due south brand certain we know what nosotros have been discussing. Biodiesel is a methyl (or ethyl) ester of a fatty acid. It is made from vegetable oil, but it is not vegetable oil. If we take 100% biodiesel, information technology is known as B100 – it is a vegetable oil that has been transesterified to brand biodiesel. It must run into ASTM biodiesel standards to qualify for warranties and sell equally biodiesel and authorize for any tax credits. About often, it is blended with petroleum-based diesel. If is B2, information technology has 2% biodiesel and 98% petroleum-based diesel fuel. Other blends include: B5 (5% biodiesel), B20 (20% biodiesel), and B100 (100% biodiesel). We'll discuss why blends are used in the post-obit section. And to exist articulate: sometimes vegetable oil is used in diesel engines, only information technology can cause performance problems and deteriorate engines over time. Sometimes, vegetable oil and booze are mixed together in emulsions, but that information technology is even so not biodiesel, as it has different properties from biodiesel.
So, if straight vegetable oil (SVO) will run in a diesel engine, why not utilize it? Vegetable oil is significantly more glutinous (gooey is a non-technical term) and has more poor combustion backdrop. It can crusade: carbon deposits, poor lubrication inside the engine, and engine clothing, and information technology has common cold starting problems. Vegetable oils have natural gums that can crusade plugging in filters and fuel injectors. And for a diesel fuel engine, the injection timing is thrown off and can cause engine knocking. There are ways to mitigate these bug, which include: one) blend with petroleum-based diesel fuel (usually < 20%), 2) preheat the oil, 3) make microemulsions with alcohols, 4) "cleft" the vegetable oil, and 5) employ the method of converting SVO into biodiesel using transesterification. Other methods are used as well, just for now, nosotros'll focus on biodiesel from transesterification. Table ix.1 shows three backdrop of No. 2 diesel, biodiesel, and vegetable oil. As you tin can see, the primary modify is in the viscosity. No. 2 diesel and biodiesel accept viscosities that are similar, merely vegetable oils take much viscosity and can cause major problems in cold weather. This is the main reason for converting the SVO into biodiesel.
| Fuel | Energy Content (Btu/gal) | Cetane Number | Viscosity (centistokes) |
|---|---|---|---|
| No. 2 Diesel | 140,000 | 48 | 3 |
| Biodiesel | 130,000 | 55 | v.7 |
| Vegetable oil | 130,000 | l | 45 |
Source: https://www.e-education.psu.edu/egee439/node/683
Posted by: hagemanhimpre.blogspot.com

0 Response to "Animal Fats And Plant Oils Differ Primarily In What Way?"
Post a Comment